Abstract
Background: Rituximab-containing chemotherapy, such as R-CHOP, is the standard initial treatment for patients (pts) with diffuse large B-cell lymphoma (DLBCL); however, 10-15% exhibit refractory or early progressive disease. Although high-dose therapy followed by autologous stem cell transplantation (HDT/ASCT) has been the standard of care for pts with relapsed or refractory DLBCL who respond to salvage chemotherapy, its role in pts refractory to initial rituximab-containing chemotherapy is not well known. Furthermore, the prognostic impact of double expression of MYC and BCL2, which has been reported as a poor prognostic factor for DLBCL pts treated with R-CHOP, in this population has not been examined.
Methods: Among all 451 newly diagnosed DLBCL pts undergoing R-CHOP as initial therapy at our institution from 2003 to 2015, we identified 69 pts (15%) with primary refractory DLBCL. Pts with primary refractory DLBCL were classified as either primary progressors (no response to R-CHOP) or partial responders (partial response to R-CHOP or relapse within 6 months after completion of R-CHOP). We analyzed the clinicopathological features and prognosis of the pts with primary refractory DLBCL. Progression-free survival (PFS) was calculated from the time of primary refractory judgment to disease progression or death from any cause, and overall survival (OS) was calculated from the time of primary refractory judgment to death from any cause.
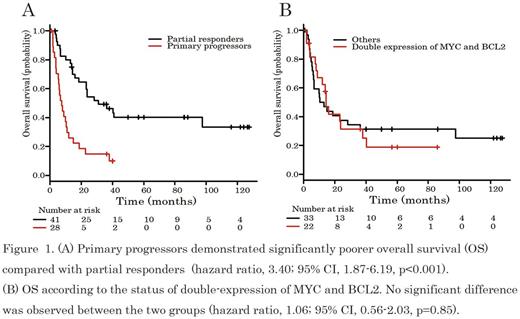
Results: The baseline characteristics at initial diagnosis of the 69 pts with primary refractory DLBCL were as follows; the median age was 64 years (range, 24-82), 33 pts (48%) were male, 55 (80%) exhibited LDH elevation, 48 (70%) had stage III or IV disease, 26 (38%) had bulky (>7.5 cm) disease, and 43 (62%) were high-intermediate or high risk pts on the International Prognostic Index (IPI). Immunohistochemically, double-expression of MYC and BCL2 was assessed in 55 out of 69 pts (80%), and confirmed in 22 pts (40%). The cell-of-origin subtype according to the Hans algorithm was assigned in 63 of the 69 pts (28 [44%] germinal-center B-cell-like [GCB] and 35 [56%] non-GCB). Twenty-eight pts (41%) were primary progressors and 41 pts (59%) were partial responders. The baseline characteristics, including age, LDH, stage, IPI risk, and the status of double-expression of MYC and BCL2, were compared between primary progressors and partial responders, and no significant differences other than in IPI risk were observed. The primary progressors included more pts with higher IPI risk than the partial responders. Sixty-five (94%) of the 69 pts received several types of salvage therapy such as systemic chemotherapy in 45 pts (69%) or local treatment in 20 pts (31%). The details of local treatments are as follows; radiotherapy (RT) to localized residual disease (10 pts), whole brain RT to solitary CNS relapse (8 pts), palliative RT and total gastrectomy in one pt each. In the 65 pts who received salvage therapy, 49% (32 pts consisted of 10 primary progressors and 22 partial responders) achieved responses, including 16 pts (25%) with complete response. The median follow-up time of the survivors was 56 months. The estimated 3-year PFS and OS were 26% (95%CI: 16-37%) and 35% (95%CI: 24-47%), respectively. Primary progressors demonstrated a significantly poorer prognosis compared with the partial responders (median OS: 7 months vs 31 months, p<0.001) (Figure 1A). Interestingly, double-expression of MYC and BCL2 had no impact on OS (Figure 1B). Finally, 17 pts (25%), 4 primary progressors and 13 partial responders, who had responded to salvage chemotherapy received HDT/ASCT. The main reasons for transplant ineligibility were advanced age (32 pts), chemo-refractory disease (12 pts), and successful local treatment (6 pts). After HDT/ASCT, 7 pts, including only 1 primary progressor, have survived without relapse for more than 3 years.
Conclusions: Our results suggest the clinical heterogeneity of primary refractory DLBCL treated with R-CHOP. Although HDT/ASCT remains a potentially curative approach, the prognosis of primary refractory DLBCL, especially in primary progressors, was extremely poor regardless of HDT/ASCT. This patient population is a candidate for investigational treatment strategies. In addition, our results suggest that the status of double-expression of MYC and BCL2 has no impact on OS in primary refractory DLBCL.
Maruyama: Chugai, Kyowa Hakko Kirin, Ono, Celgene, Janssen, Eisai, Mundipharma, Takeda: Honoraria; Dai-ichi Sankyo, Chugai, Kyowa Hakko Kirin, Ono, Celgene, Janssen, GSK, Eisai, Mundipharma, Takeda, AbbVie, MSD, Sanofi, Pfizer, Otsuka, Novartis, Solasia, Zenyaku: Research Funding. Makita: Celgene, Chugai: Honoraria. Kobayashi: Pfizer, Ohtsuka, Astellas, Ariad: Research Funding. Izutsu: Abbvie, Astellas, Astellas Amgen, Bayer, Celgene, Celltrion, Chugai, Daiichi Sankyo, Eisai, HUYA Bioscience International, Janssen Edit, Mundhi, Novartis, Ono, Sanofi, Solasia, Symbio, Takeda, Zenyaku: Research Funding; Chugai, Eisai, Gilead, Janssen Edit, Kyowa Hakko Kirin, MSD, Ono, Takeda: Honoraria; Celgene, Bayer: Consultancy. Tobinai: Servier: Research Funding; Janssen: Honoraria, Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; HUYA Bioscience: Honoraria; AbbVie: Research Funding; Mundipharma: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Eisai: Honoraria, Research Funding; Daiichi Sankyo Co., Ltd: Consultancy, Honoraria; Ono Pharmaceutical: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Zenyaku Kogyo: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal